Abstract
Introduction: Luspatercept, a TGF-B fusion trap protein, is a first in class erythroid maturating agent approved by FDA for treatment of red blood cell transfusion dependent (RBC-TD) lower risk myelodysplastic syndromes (LR-MDS) with ring sideroblasts (RS) based on red blood cell transfusion independence (RBC-TI) in 38% of 153 patients (pts) in the MEDALIST study. Erythroid stimulating agents (ESA) promote early stages of erythropoiesis while luspatercept enhances terminal erythroid maturation, thus the combination of ESA and luspatercept may have synergistic effect. We report data on the activity of combining luspatercept with ESA.
Methods We treated a cohort of pts off-protocol at Moffitt Cancer Center with ESA and luspatercept combination if no response/loss of response was observed to luspatercept monotherapy as add back strategy or upfront combination. Baseline RBC transfusion burden (TB) was defined as: non- transfusion dependent (NTD) (0 units in 8 weeks prior luspatercept), low TB (LTB) (1-5 units /8weeks) and high TB (HTB) (≥ 6 units /8 weeks). A hematological response (HI) was defined as (1) an objective Hgb increase of > 1.5 g/dl in NTD, (2) RBC-TI with Hgb increase of 1.5 g/dl, or RBC-TI without Hgb 1.5 g/dl increase or >50% reduction in RBC transfusion burden among RBC-TD. Luspatercept dose escalation and choice of ESA/dosing was at the discretion of the treating physician.
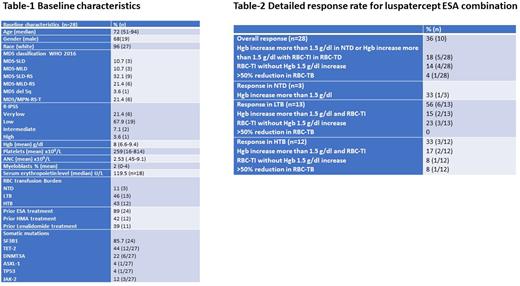
Results Between February 2020 and September 2021, 28 pts were treated with luspatercept and ESA combination after no response (1º failure) (n=18) or loss of initial response to luspatercept monotherapy (2º failure) (n=7) or as initial combination treatment (n=3). Table-1 summarizes baseline characteristics. The median age was 72 years and 53.5% had MDS-RS subtype; 96.4% were intermediate or lower risk MDS by IPSS-R. SF3B1 somatic mutation was detected in 85.7%. The mean Hgb level was 8 g/dl and 43% were RBC HTB transfusion dependent. The median serum erythropoietin (epo) level available for 18 pts at time referral was 119.5 U/L. The majority had prior ESA treatment 89% (25) with 21.4% (6/25) hematological response to prior ESA monotherapy. Additionally, 12 pts (43%) had prior HMA and 11 pts (39%) had prior lenalidomide (Len) treatment.
The concurrent ESA was epoetin in 16 pts and darbepoetin in 12 pts. The majority of pts (27/28; 96%) had luspatercept dose escalation whereby 23 pts received 1.33 mg/kg, 25 pts received 1.75 mg, and only 1 pt did not receive any luspatercept dose escalation.
The overall HI rate to luspatercept combined with ESA was 36% (10/28). Table-2 summarizes detailed responses. HI according to baseline RBC-TB were observed in 33% (1/3) NTD, 56% (6/13) LTB and 25% HTB (3/12) pts respectively, p=.75. Five out 7 pts (71%) who responded originally to luspatercept alone (2º failure) responded to ESA add on whereas only 3 out of 18 pts (17%) who did not respond to luspatercept monotherapy (1º failure) responded when ESA was added. With upfront ESA + luspatercept combination, 2 out 3 pts responded.
Among responders, the median duration of response from time of response to luspatercept monotherapy or combination was 18.9 months (5.4-27.3). As of last follow up, 15 pts (54%) discontinued treatment. Reason of discontinuation was lack of response in 12 pts and loss of response in 3 pts.
No responses to combination ESA/luspatercept were observed among SF3B1 wild type pts (n=4). Baseline serum epo levels were available for 18 pts at time of referral, no pts with serum epo > 500 u/l responded to addition of ESA, 25% of pts (2/8) with serum epo 200-500 u/l responded and 40% of pts (4/10) with serum epo less than 200 U/L responded.
There was a trend of higher response among HMA and Len naïve pts. The HI rate was 25% (3/12) after HMA failure compared to 44% (7/16) in HMA naïve pts (p=.3). However, more pts post HMA failure were RBC-HTB 67% (8/12) compared to 25% (4/16) in HMA naïve, p=.05. The HI rate was 18% (2/11) for Len failure compared to 47% (8/17) Len naïve pts, (p=.1). No difference in HTB was observed among lenalidomide naïve and treated patients.
Conclusions To our knowledge, this is the first proof of principle data confirming synergistic clinical activity combining luspatercept and ESA. Predictors of response included prior response to luspatercept monotherapy/frontline combination compared to primary luspatercept failure, endogenous serum epo levels < 500, SF3B1 mutation, and being HMA/Len treatment naïve.
Disclosures
Komrokji:CTI biopharma: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Taiho: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Geron: Honoraria, Membership on an entity's Board of Directors or advisory committees; PharmaEssentia: Honoraria, Other, Speakers Bureau; Servio: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Jazz: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Kuykendall:Pharmaessentia: Consultancy, Honoraria, Speakers Bureau; Imago Biosciences: Consultancy, Honoraria, Speakers Bureau; Incyte: Consultancy, Honoraria, Speakers Bureau; Blueprint: Consultancy, Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria, Speakers Bureau; Abbvie: Consultancy, Honoraria, Speakers Bureau; GSK - Sierra Oncology: Consultancy, Honoraria, Other: Research Support, Speakers Bureau; Prelude Pharmaceuticals: Other: Research Support; BMS: Consultancy, Honoraria, Other: Research Support, Speakers Bureau; Morphosys: Other: Research Support; Protagonist: Other: Research Support; CTI Biopharma: Consultancy, Honoraria, Speakers Bureau. Sweet:Gilead Sciences, Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Astellas: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Curis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Research Funding; berGenBio: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Mablytics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Syntrix Pharmaceuticals: Research Funding; AROG: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Lancet:Novartis: Consultancy; Boxer Capital: Consultancy; Dava Oncology: Consultancy; Syntrix Pharmaceuticals: Research Funding; Dedham Group: Consultancy; Jasper Therapeutics: Consultancy; Astellas: Consultancy; Agios/Servio: Consultancy; Jazz: Consultancy; BerGenBio: Consultancy; Millenium Pharma/Takeda: Consultancy; ElevateBio Management: Consultancy; Daiichi Sankyo: Consultancy; Celgene/BMS: Research Funding; AbbVie: Consultancy; Servier: Consultancy. Padron:BMS: Research Funding; Stemline: Honoraria; Taiho: Honoraria; Blueprint: Honoraria; Kura: Research Funding; Incyte: Research Funding; Syntrix Pharmaceuticals: Research Funding. Sallman:Syndax: Membership on an entity's Board of Directors or advisory committees; Intellia: Membership on an entity's Board of Directors or advisory committees; Incyte: Speakers Bureau; Syntrix Pharmaceuticals: Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Nemucore: Membership on an entity's Board of Directors or advisory committees; Magenta: Consultancy; BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AbbVie: Membership on an entity's Board of Directors or advisory committees; Aprea: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Consultancy; Agios: Membership on an entity's Board of Directors or advisory committees; Kite: Membership on an entity's Board of Directors or advisory committees; Lixte: Patents & Royalties: LB-100; Shattuck Labs: Membership on an entity's Board of Directors or advisory committees.
OffLabel Disclosure:
combination using erythroid stimulating agents and luspatercept
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal